What is a Catalyst? Definition and Role in Forensic Science
A catalyst is a substance that increases the rate of a chemical reaction without itself being consumed or permanently altered in the process. It works by providing an alternative reaction pathway with a lower activation energy, thereby making it easier and faster for reactants to form products. In biology, proteins that act as catalysts are known as enzymes.
How Catalysts Work
Think of a catalyst as a helper molecule. It participates in the reaction to lower the energy barrier but is regenerated at the end, allowing a single catalyst molecule to facilitate many reaction cycles. This is a fundamental concept in chemistry, controlling everything from industrial manufacturing to the biological processes in our own cells.
The Role of Catalysts in Forensic Science
While a basic chemistry concept, catalysis is the principle behind several essential forensic techniques:
Arson Investigation: While not a chemical test, the catalytic converter in a vehicle can be a source of investigation. These devices operate at extremely high temperatures and can act as an ignition source for flammable liquids or vapors, a factor that must be considered when reconstructing a fire scene.
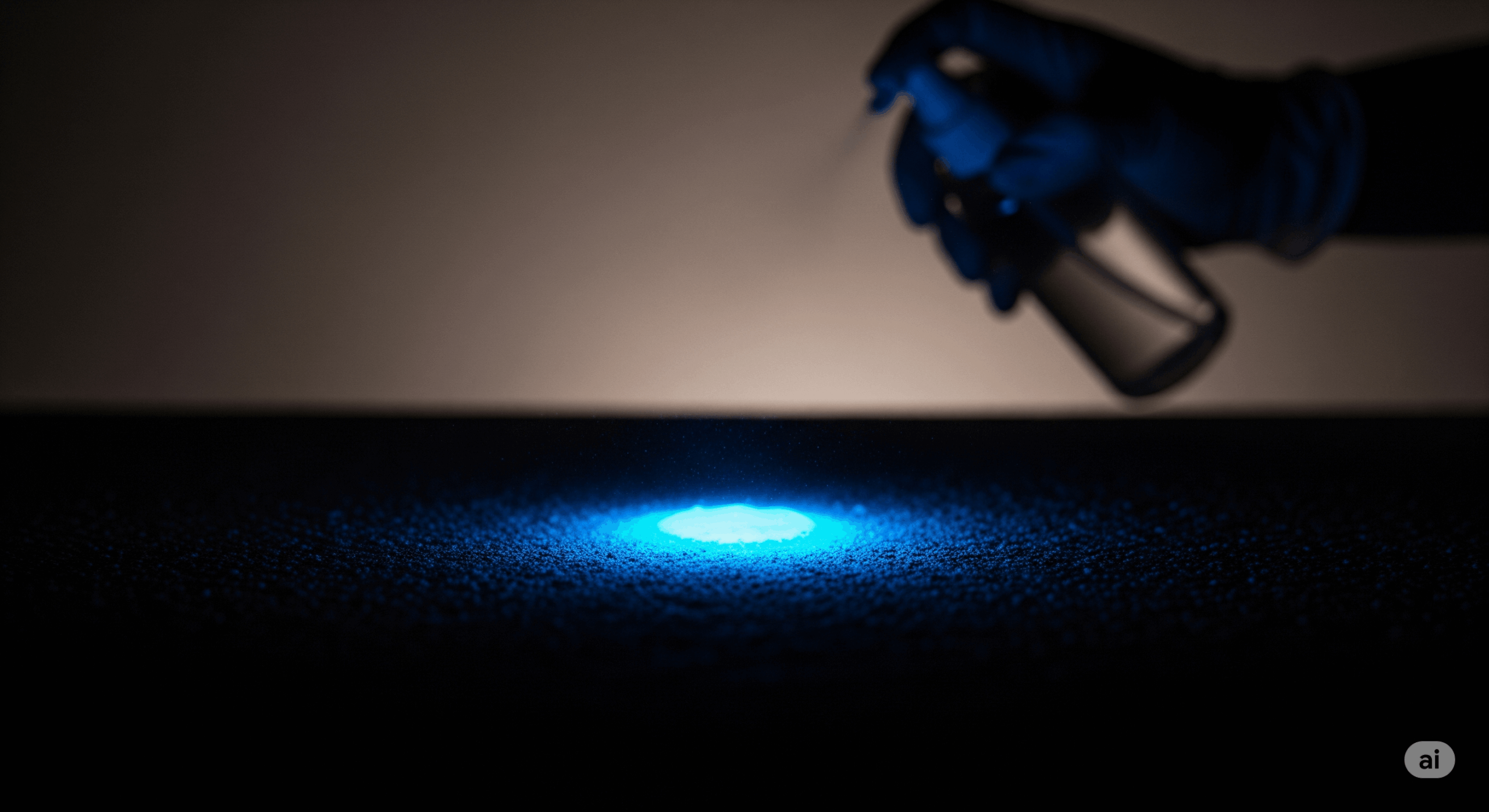
Luminol Test for Blood: This is the classic example. The iron within the heme group of hemoglobin in blood acts as a powerful catalyst for the chemiluminescent reaction of luminol. When sprayed on a surface with latent bloodstains, the iron catalyzes the reaction, causing luminol to emit a characteristic blue glow, revealing the pattern.
Presumptive Drug Tests: Many field tests for drugs utilize specific catalysts to accelerate a color-change reaction. A reagent will change color in the presence of a specific drug, and a catalyst ensures that this reaction occurs quickly enough to be observed on the spot.